Islet Cell Response to High Fat Programming in Neonate, Weanling and Adolescent Wistar Rats
Abstract
Context High fat programming, by exposure to a high saturated fat diet during fetal and/or lactational life induces metabolic derangements and alters islet cell architecture in neonate and weanling rats. Objective The present study assessed metabolic changes and islet cell dynamics in response to high fat maintenance during specific developmental periods in adolescent rats, with some parameters also studied in neonate and weanling rats. Methods The experimental groups comprised neonates, weanlings and adolescents maintained on a high fat diet during specific periods of fetal, lactational and/or postnatal life. Control neonates, weanlings and adolescents were maintained on a standard laboratory (control or low fat) diet. Fetal high fat programmed (i.e., maintained on a high fat diet exclusively during fetal life) neonates were insulin resistant. Results Weanlings maintained on a high fat diet throughout fetal and lactational life had increased pancreas weights. Fetal high fat programmed adolescents presented a normal phenotype mimicking the control adolescents. Adolescents maintained on a postnatal high fat diet had increased body weights, hyperglycemia, hyperinsulinemia, hyperleptinemia and insulin resistance displaying beta cell hypertrophy and increased islet cell proliferation. Adolescents maintained on a fetal and postnatal high fat diet had increased body weights, hyperleptinemia, hyperinsulinemia and insulin resistance. Conclusions High fat programming induces various diabetogenic phenotypes which present at different life stages. The postnatal period from birth to adolescence represents an extension for high fat programming of metabolic disease.
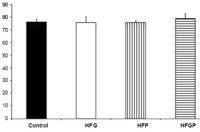
Image: Beta cell volume in adolescent rats
Downloads
References
Carolan PJ, Melton DA: New findings in pancreatic and intestinal endocrine development to advance regenerative medicine. Curr Opin Endocrinol Diabetes Obes 2013; 20:1-7. [PMID:23249759]
Gittes GK: Developmental biology of the pancreas: a comprehensive review. Dev Biol 2009; 326:4-35. [PMID:19013144]
Miralles F, Battelino T, Czernichow P, Scharfmann R: TGF-beta plays a key role in morphogenesis of the pancreatic islets of Langerhans by controlling the activity of the matrix metalloproteinase MMP-2. J Cell Biol 1998; 143:827-836. [PMID:9813100]
Kaung HL: Growth dynamics of pancreatic islet cell populations during fetal and neonatal development of the rat. Dev Dyn 1994; 200:163-75. [PMID:7919502]
Reusens B, Theys N, Dumortier O, Goosse K, Remacle C: Maternal malnutrition programs the endocrine pancreas in progeny. Am J Clin Nutr 2011; 94:1824S-1829S. [PMID:21562089]
Wu Y, Wu T, Wu J, Zhao L, Li Q, Varghese Z, Moorhead JF, Powis SH, Chen Y, Ruan XZ: Chronic inflammation exacerbates glucose metabolism disorders in C57BL/6J mice fed with high-fat diet. J Endocrinol 2013; 219:195-204. [PMID:24029730]
Collins SC, Hoppa MB, Walker JN, Amisten S, Abdulkhader F, Bengtsson M, Fearnside J, Ramracheya R, Toye AA, Zhang Q, Clark A, Gauguier D, Rorsman P: Progression of diet-induced diabetes in C57Bl6J mice involves functional dissociation of Ca2+ channels from secretory vesicles. Diabetes 2010; 59:1192-1201. [PMID:20150285]
Cerf ME, Williams K, Nkomo XI, Muller CJ, Du Toit DF, Louw J, Wolfe-Coote SA: Islet cell response in the neonatal rat after exposure to a high-fat diet during pregnancy. Am J Physiol Regul Integr Comp Physiol 2005; 288:R1122-R1128. [PMID:15705804]
Cerf ME, Chapman CS, Muller CJ, Louw J: Gestational high-fat programming impairs insulin release and reduces Pdx-1 and glucokinase immunoreactivity in neonatal Wistar rats. Metabolism 2009; 58:1787-1792. [PMID:19604517]
Cerf ME, Muller CJ, Du Toit DF, Louw J, Wolfe-Coote SA: Hyperglycaemia and reduced glucokinase expression in weanling offspring from dams maintained on a high-fat diet. Br J Nutr 2006; 95:391-396. [PMID:16469158]
Cerf ME, Williams K, Chapman CS, Louw J: Compromised beta-cell development and beta-cell dysfunction in weanling offspring from dams maintained on a high-fat diet during gestation. Pancreas 2007; 34:347-353. [PMID:17414058]
Cerf ME, Louw J: High fat programming induces glucose intolerance in weanling Wistar rats. Horm Metab Res 2010; 42:307-310. [PMID:20195946]
Cerf ME, Chapman CS, Louw J: High-fat programming of hyperglycemia, hyperinsulinemia, insulin resistance, hyperleptinemia, and altered islet architecture in 3-month-old wistar rats. ISRN Endocrinol 2012; 2012:627270. [PMID:22988521]
Tichelaar HY, Spinnler Benade AJ, Daubitzer AK, Kotze TJ: An improved rapid thin-layer chromatographic-gas-liquid chromatographic procedure for the determination of free fatty acids in plasma. Clin Chim Acta 1989; 183:207-215. [PMID:2791305]
Portha B, Chavey A, Movassat J: Early-life origins of type 2 diabetes: fetal programming of the beta-cell mass. Exp Diabetes Res 2011; 2011:105076. [PMID:22110471]
Blandino-Rosano M, Alejandro EU, Sathyamurthy A, Scheys JO, Gregg B, Chen AY, Rachdi L, Weiss A, Barker DJ, Gould AP, Elghazi L, Bernal-Mizrachi E: Enhanced beta cell proliferation in mice overexpressing a constitutively active form of Akt and one allele of p21Cip. Diabetologia 2012; 55:1380-1389. [PMID:22327314]
Dunning BE, Gerich JE: The role of alpha-cell dysregulation in fasting and postprandial hyperglycemia in type 2 diabetes and therapeutic implications. Endocr Rev 2007; 28:253-283. [PMID:17409288]
Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA: In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature 2008; 455:627-632. [PMID:18754011]
Tushuizen ME, Bunck MC, Pouwels PJ, Bontemps S, van Waesberghe JH, Schindhelm RK, Mari A, Heine RJ, Diamant M: Pancreatic fat content and beta-cell function in men with and without type 2 diabetes. Diabetes Care 2007; 30:2916-2921. [PMID:17666465]
Scaglia L, Cahill CJ, Finegood DT, Bonner-Weir S: Apoptosis participates in the remodeling of the endocrine pancreas in the neonatal rat. Endocrinology 1997; 138:1736-1741. [PMID:9075738]
Bouwens L, Rooman I: Regulation of pancreatic beta-cell mass. Physiol Rev 2005; 85:1255-1270. [PMID:16183912]
Liu Y, Shi S, Gu Z, Du Y, Liu M, Yan S, Gao J, Li J, Shao Y, Zhong W, Chen X, Li C: Impaired autophagic function in rat islets with aging. Age (Dordr) 2013; 35:1531-1544. [PMID:22843415]
Ackermann AM, Gannon M: Molecular regulation of pancreatic beta-cell mass development, maintenance, and expansion. J Mol Endocrinol 2007; 38:193-206. [PMID:17293440]
Unger RH, Orci L: Paracrinology of islets and the paracrinopathy of diabetes. Proc Natl Acad Sci USA 2010; 107:16009-16012. [PMID:20798346]
Mehran AE, Templeman NM, Brigidi GS, Lim GE, Chu KY, Hu X, Botezelli JD, Asadi A, Hoffman BG, Kieffer TJ, Bamji SX, Clee SM, Johnson JD: Hyperinsulinemia drives diet-induced obesity independently of brain insulin production. Cell Metab 2012; 16:723-737. [PMID:23217255]
Ghezzi AC, Cambri LT, Botezelli JD, Ribeiro C, Dalia RA, Rostom de Mello MA: Metabolic syndrome markers in wistar rats of different ages. Diabetol Metab Syndr 2012; 4:16. [PMID:23249759]
Hoppa MB, Collins S, Ramracheya R, Hodson L, Amisten S, Zhang Q, Johnson P, Ashcroft FM, Rorsman P: Chronic palmitate exposure inhibits insulin secretion by dissociation of Ca(2+) channels from secretory granules. Cell Metab 2009; 10:455-465. [PMID:19945403]
Weir GC, Bonner-Weir S: Islet beta cell mass in diabetes and how it relates to function, birth, and death. Ann NY Acad Sci 2013; 1281:92-105. [PMID:23363033]
Kasuga M: Insulin resistance and pancreatic beta cell failure. J Clin Invest 2006;116:1756-60. [PMID:16823472]
Zhang Y, Xiao M, Niu G, Tan H: Mechanisms of oleic acid deterioration in insulin secretion: role in the pathogenesis of type 2 diabetes. Life Sci 2005; 77:2071-2081. [PMID:15935394]
Copyright (c) 2014 Marlon E Cerf, Johan Louw

This work is licensed under a Creative Commons Attribution 4.0 International License.
As a member of Publisher International Linking Association, PILA, iMedPub Group’s JOP follows the Creative Commons Attribution License and Scholars Open Access publishing policies. Journal of the Pancreas is the Council Contributor Member of Council of Science Editors (CSE) and following the CSE slogan Education, Ethics, and Evidence for Editors.