Ototoxicity Associated with Oxaliplatin in a Patient with Pancreatic Cancer
Abstract
Context Oxaliplatin, a third-generation platinum derivative is commonly used for the treatment of colorectal cancer, pancreatic cancer, upper gastrointestinal cancer, hepatobiliary cancer, and ovarian cancer. Neurotoxicity is the dose limiting toxicity and ototoxicity is very rare, less than 1% of patients. Case report We present a case of a female patient with locally advanced unresectable pancreatic cancer who developed hearing loss after receiving oxaliplatin and gemcitabine. The dose of oxaliplatin was reduced but continued due to clinical benefit and radiological response. Discussion To the best of our knowledge, this is the third case report of oxaliplatin-induced ototoxicity. Ototoxicity seems to be a rare complication of oxaliplatin therapy. Regardless of its rare occurrence, clinicians should be aware of this severe complication and be diligent in monitoring patients’s clinical symptoms.
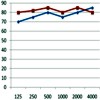
Image: Post-oxaliplatin audiogram.
Downloads
References
Ibrahim A, Hirschfeld S, Cohen MH, Griebel DJ, Williams GA, Pazdur R. FDA drug approval summaries: oxaliplatin. Oncologist. 2004;9(1):8-12.
Hoff PM, Saad ED, Costa F, Coutinho AK, Caponero R, Prolla G, Gansl RC. Literature review and practical aspects on the management of oxaliplatin-associated toxicity Clin Colorectal Cancer. 2012 Jun;11(2):93-100
Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C, Bennouna J, Bachet JB, Khemissa-Akouz F, Péré-Vergé D, Delbaldo C, Assenat E, Chauffert B, Michel P, Montoto-Grillot C, Ducreux M; Groupe Tumeurs Digestives of Unicancer; PRODIGE Intergroup. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011 May 12;364(19):1817-25
Li J, Merl M, Lee MX, Kaley K, Saif MW. Safety and efficacy of single-day GemOx regimen in patients with pancreatobiliary cancer: a single institution experience. Expert Opin Drug Saf. 2010 Mar;9(2):207-13.
Afchain P, Chibaudel B, Lledo G, Selle F, Bengrine-Lefevre L, Nguyen S, Paitel JF, Mineur L, Artru P, André T, Louvet C. First-line simplified GEMOX (S-GemOx) versus classical GEMOX in metastatic pancreatic cancer (MPA): results of a GERCOR randomized phase II study. Bull Cancer. 2009 May;96(5):E18-22.
Saif MW, Reardon J. Management of oxaliplatin-induced peripheral neuropathy. Ther Clin Risk Manag. 2005 Dec;1(4):249-58.
Saif MW. Oral Calcium Ameliorating Oxaliplatin-Induced Peripheral Neuropathy. J Appl Res. 2004 Jan 1;4(4):576-582.
Saif MW, Kim R. Role of platinum agents in the management of advanced pancreatic cancer. Expert Opin Pharmacother. 2007 Nov;8(16):2719-27.
Hellberg V, Wallin I, Eriksson S, Hernlund E, Jerremalm E, Berndtsson M, Eksborg S, Arnér ES, Shoshan M, Ehrsson H, Laurell G. Cisplatin and oxaliplatin toxicity: importance of cochlear kinetics as a determinant for ototoxicity. J Natl Cancer Inst. 2009 Jan 7;101(1):37-47
Ding D, Allman BL, Salvi R. Review: ototoxic characteristics of platinum antitumor drugs . Anat Rec (Hoboken). 2012 Nov;295(11):1851-67
Malhotra NK, Aslam R, Lipman SP, Bilski VJ. Acute ototoxicity from a single infusion of oxaliplatin. Ear Nose Throat J. 2010 Jun;89(6):258-61
Vietor NO, George BJ. Oxaliplatin-induced hepatocellular injury and ototoxicity: a review of the literature and report of unusual side effects of a commonly used chemotherapeutic agent. J Oncol Pharm Pract. 2012 Sep;18(3):355-9.
Cassidy J, Misset JL Oxaliplatin-related side effects:characteristics and management. Semin Oncol. 2002 Oct;29(5 Suppl 15):11-20.
Copyright (c) 2014 Sun Young Oh, Nawal Wasif, Marie Carmel Garcon, Gladys Rodriguez, Muhammad Wasif Saif

This work is licensed under a Creative Commons Attribution 4.0 International License.
As a member of Publisher International Linking Association, PILA, iMedPub Group’s JOP follows the Creative Commons Attribution License and Scholars Open Access publishing policies. Journal of the Pancreas is the Council Contributor Member of Council of Science Editors (CSE) and following the CSE slogan Education, Ethics, and Evidence for Editors.