Neoadjuvant Chemo-Radiotherapy for Patients with Borderline Resectable Pancreatic Cancer: A Meta-Analytical Evaluation of Prospective Studies
Abstract
Context For patients with borderline resectable pancreatic cancer, the benefit of neoadjuvant therapy remains to be defined. Objective We did a systematic search of the literature on this topic. Methods Prospective studies where chemotherapy with or without radiotherapy was given before surgery to patients with borderline resectable cancer, were analyzed by a meta-analytical approach. Main outcome measures Primary outcome was surgical exploration and resection rates; tumor response, therapy-induced toxicity, and survival were secondary outcomes. Data were expressed as weighted pooled proportions with 95% confidence intervals (95% CI). Results Ten studies with 182 participants were included. Following treatment, 69% of patients (95% CI: 56-80%) were brought to surgery and 80% (95% CI: 66-90%) of surgically-explored patients were resected. Eighty-three percent (95% CI: 74-90%) of resected specimens were deemed R0 resections. The weighted fractions of resected patients alive at 1 and 2 years were 61% (95% CI: 48-100%) and 44% (95% CI: 32-59%), respectively. At restaging following neoadjuvant therapy, weighted frequencies for complete/partial response were 16% (95% CI: 9-28%), 69% (95% CI: 60-76%) for stable disease, and 19% (95% CI: 13-25%) for progressive cancer. Treatment-related grade 3-4 toxicity was 32% (95% CI: 21-45%). Conclusion This meta-analysis shows that downstaging of the lesion following neoadjuvant therapies is uncommon for patients with borderline resectable pancreatic cancer. A clear benefit of this regimen could be to spare surgery to patients with progressive disease during the frame-time chemo-radiotherapy is being delivered.
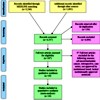
Image: PRISMA 2009 flow diagram.
Downloads
References
Varadhachary GR, Tamm EP, Abbruzzese JL, Xiong HQ, Crane CH, Wang H, et Al. Borderline resectable pancreatic cancer: definitions, management, and role of preoperative therapy. Ann Surg Oncol 2006;13:1035- 46.
Callery MP, Chang KJ, Fishman EK, Talamonti MS, William Traverso L, Linehan DC. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: expert consensus statement. Ann Surg Oncol 2009;16:1727-33
National Comprehensive Cancer Network (NCCN) guidelines. Available at: www.nccn.org (Accessed on October 13, 2011).
Gillen S, Schuster T, Meyer Zum Büschenfelde C, Friess H, Kleeff J. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med 2010;7(4):e1000267
Andriulli A, Festa V, Botteri E, Valvano MR, Koch M, Bassi C, et Al.Neoadjuvant/Preoperative Gemcitabine for Patients with Localized Pancreatic Cancer: A Meta-analysis of Prospective Studies. Ann Surg Oncol 2012;19:1644-62
Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta-analyses. Lancet 1999;354:1896–1900
Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097
Morley PT, Atkins DL, Billi JE, Bossaert L, Callaway CW, de Caen AR, et Al. Part 3: Evidence evaluation process: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation. 2010;122 (16 Suppl 2):S283-90
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et Al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000,92:205-216
Wilson E.B. Probable inference, the law of succession, and statistical inference. J Am Stat Ass 1927; 22: 209–212
Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev. 1987; 9:1-30
Higgings JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539-1558
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629-634
Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101
van Houwelingen HC, Arends LR, Stijnen T. Advanced methods in meta-analysis: multivariate approach and meta-regression. Stat Med 2002;21:589–624
Mehta VK, Fisher G, Ford JA, Poen JC, Vierra MA, Oberhelman H, et al. Preoperative chemoradiation for marginally resectable adenocarcinoma of the pancreas. J Gastrointest Surg 2001;5:27-35
Magnin V, Moutardier V, Giovannini MH, Lelong B, Giovannini M, Viret F, et Al. Neoadjuvant preoperative chemoradiation in patients with pancreatic cancer. Int J Radiat Oncol Biol Phys 2003;55:1300-4
Pipas JM, Barth RJ Jr, Zaki B, Tsapakos MJ, Suriawinata AA, Bettmann MA, et Al. Docetaxel/Gemcitabine followed by gemcitabine and external beam radiotherapy in patients with pancreatic adenocarcinoma. Ann Surg Oncol 2005;12:995-1004
Massucco P, Capussotti L, Magnino A, Sperti E, Gatti M, Muratore A, et Al. Pancreatic resections after chemoradiotherapy for locally advanced ductal adenocarcinoma: analysis of perioperative outcome and survival. Ann Surg Oncol 2006;13:1201-8
Le Scodan R, Mornex F, Girard N, Mercier C, Valette PJ, Ychou M, et Al. Preoperative chemoradiation in potentially resectable pancreatic adenocarcinoma: feasibility, treatment effect evaluation and prognostic factors, analysis of the SFRO-FFCD 9704 trial and literature review. Ann Oncol 2009;20:1387-96
Leone F, Gatti M, Massucco P, Colombi F, Sperti E, Campanella D, et Al. Induction gemcitabine and oxaliplatin therapy followed by a twice-weekly infusion of gemcitabine and concurrent external-beam radiation for neoadjuvant treatment of locally advanced pancreatic cancer: A single institutional experience. Cancer. 2012 Jul 6. doi: 10.1002/cncr.27736. [Epub ahead of print]
Small W Jr, Mulcahy MF, Rademaker A, Bentrem DJ, Benson AB, Weitner BB, et Al. Phase II trial of full-dose gemcitabine and bevacizumab in combination with attenuated three-dimensional conformal radiotherapy in patients with localized pancreatic cancer. Int J Radiat Oncol Biol Phys 2011;80:476-82
Sahora K, Kuehrer I, Schindl M, Koelblinger C, Goetzinger P, Gnant M. NeoGemTax: gemcitabine and docetaxel as neoadjuvant treatment for locally advanced nonmetastasized pancreatic cancer. World J Surg 2011;35:1580-9
Sahora K, Kuehrer I, Eisenhut A, Akan B, Koellblinger C, Goetzinger P, et Al. NeoGemOx: Gemcitabine and oxaliplatin as neoadjuvant treatment for locally advanced, nonmetastasized pancreatic cancer. Surgery 2011;149:311-20
Lee JL, Kim SC, Kim JH, Lee SS, Kim TW, Park DH, et Al. Prospective efficacy and safety study of neoadjuvant gemcitabine with capecitabine combination chemotherapy for borderline-resectable or unresectable locally advanced pancreatic adenocarcinoma. Surgery 2012 Jun 6. [Epub ahead of print]
Assifi MM, Lu X, Eibl G, Reber HA, Li G, Hines OJ. Neoadjuvant therapy in pancreatic adenocarcinoma: a meta-analysis of phase II trials. Surgery 2011;150:466-73
Katz MHG, Fleming JB, Bhosale P, Varadhachary G, Lee JE, Wolff R, et Al. Response of borderline resectable pancreatic cancer to neoadjuvant therapy is not reflected by radiographic indicators. Cancer 2012 May 17. [Epub ahead of print]
Zhang Y, Huang J, Chen M, Jiao LR. Preoperative vascular evaluation with computed tomography and magnetic resonance imaging for pancreatic cancer: A meta-analysis. Pancreatology 2012;12:227-33.
Copyright (c) 2014 Virginia Festa, Angelo Andriulli, Maria Rosaria Valvano, Generoso Uomo, Francesco Perri, Nicola Andriulli, Salvatore Corrao, Maurizio Koch

This work is licensed under a Creative Commons Attribution 4.0 International License.
As a member of Publisher International Linking Association, PILA, iMedPub Group’s JOP follows the Creative Commons Attribution License and Scholars Open Access publishing policies. Journal of the Pancreas is the Council Contributor Member of Council of Science Editors (CSE) and following the CSE slogan Education, Ethics, and Evidence for Editors.