Differential Expression of GNAS and KRAS Mutations in Pancreatic Cysts
Abstract
Context KRAS mutations play an important role in pancreatic cancer. GNAS mutations were discovered in intraductal papillary mucinous neoplasms (IPMN). Objectives Our aim was to identify the frequency of KRAS and GNAS mutations in pancreatic cystic neoplasms and pancreatic ductal adenocarcinoma (PDAC). Methods Sixty-eight surgically resected formalin fixed, paraffin embedded pancreatic specimens were analyzed, including: 1) benign (20 serous cystadenoma (SCA)), 2) pre-malignant (10 mucinous cystic neoplasm (MCN), 10 branch duct intraductal papillary mucinous neoplasm (BD-IPMN), 9 main duct IPMN (MD-IPMN)), 3) malignant (19 PDAC). Total nucleic acid extraction was performed. KRAS codon 12/13 and GNAS codon 201 mutations were interrogated via targeted sequencing using the Ion Torrent's Personal Genome Machine (PGM). Results Mean age of 68 patients was 61.9±8.4 with 72% female. KRAS and GNAS mutations were more common in PDAC and IPMN. KRAS mutations predominated in PDAC compared to pancreatic cysts (16/19, 84% versus 10/49, 20%; P<0.001). GNAS mutations were more common in IPMN compared to non-IPMN lesions (8/19, 42% versus 2/49, 4%; P=0.0003). No GNAS mutations were detected in PDAC and MCN while 2 SCA carried GNAS mutations. Double mutations with KRAS and GNAS were only present in IPMN (5/19 versus 0/30 SCA and MCN, P=0.006). Conclusions KRAS and GNAS mutations were more common in PDAC and IPMN with KRAS mutations primarily in PDAC and GNAS mutations more frequent in IPMN. No GNAS mutations occurred in MCN and double mutations were only present in IPMN.
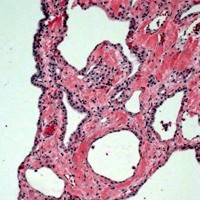
Image: Serous cystadenoma with microcysts lined by bland cuboidal epithelium.
Downloads
References
Simeone DM. SSAT/AGA/ASGE state of the art conference on cystic neoplasms of the pancreas. J Gastrointest Surg. Aug 2008; 12: 1475-1477.[PMID: 18224378]
Fasanella KE, McGrath K. Cystic lesions and intraductal neoplasms of the pancreas. Best Pract Res Clin Gastroenterol. 2009; 23: 35-48. [PMID: 19258185]
Lee LS, Clancy T, Kadiyala V, Suleiman S, Conwell DL. Interdisciplinary management of cystic neoplasms of the pancreas. Gastroenterol Res Pract. 2012; 2012: 513163. [PMID: 23133446]
lee LS. Evaluation and management of pancreatic cystic lesions. J Clin Outcomes Manage. 2013; 20: 129-142
Khalid A, Zahid M, Finkelstein SD, et al. Pancreatic cyst fluid DNA analysis in evaluating pancreatic cysts: a report of the PANDA study. Gastrointest Endosc. May 2009; 69: 1095-1102. [PMID: 19152896]
Wu J, Matthaei H, Maitra A, et al. Recurrent GNAS mutations define an unexpected pathway for pancreatic cyst development. Sci Transl Med. 2011; 3: 92ra66.[PMID: 21775669]
Furukawa T, Kuboki Y, Tanji E, et al. Whole-exome sequencing uncovers frequent GNAS mutations in intraductal papillary mucinous neoplasms of the pancreas. Sci Rep. 2011;1 :161.[PMID: 22355676]
Kloppel G, Luttges J. WHO-classification 2000: exocrine pancreatic tumors. Verh Dtsch Ges Pathol. 2001; 85: 219-228.[PMID: 11894402]
Hadd AG, Houghton J, Choudhary A, et al. Targeted, high-depth, next-generation sequencing of cancer genes in formalin-fixed, paraffin-embedded and fine-needle aspiration tumor specimens. J Mol Diagn. Mar 2013; 15: 234-247. [PMID: 23321017]
Correa-Gallego C, Ferrone CR, Thayer SP, Wargo JA, Warshaw AL, Fernandez-Del Castillo C. Incidental pancreatic cysts: do we really know what we are watching? Pancreatology. 2010; 10: 144-150. [PMID: 20484954]
Brugge WR, Lewandrowski K, Lee-Lewandrowski E, et al. Diagnosis of pancreatic cystic neoplasms: a report of the cooperative pancreatic cyst study. Gastroenterology. May 2004; 126: 1330-1336. [PMID: 15131794]
Lee LS, Saltzman JR, Bounds BC, Poneros JM, Brugge WR, Thompson CC. EUS-guided fine needle aspiration of pancreatic cysts: a retrospective analysis of complications and their predictors. Clin Gastroenterol Hepatol. Mar 2005; 3: 231-236. [PMID: 15765442]
Maker AV, Lee LS, Raut CP, Clancy TE, Swanson RS. Cytology from pancreatic cysts has marginal utility in surgical decision-making. Ann Surg Oncol. Nov 2008; 15: 3187-3192. [PMID: 18766406]
van der Waaij LA, van Dullemen HM, Porte RJ. Cyst fluid analysis in the differential diagnosis of pancreatic cystic lesions: a pooled analysis. Gastrointest Endosc. Sep 2005; 62: 383-389. [PMID: 16111956]
Sakorafas GH, Smyrniotis V, Reid-Lombardo KM, Sarr MG. Primary pancreatic cystic neoplasms revisited. Part I: serous cystic neoplasms. Surg Oncol. Jun 2011; 20: e84-92. [PMID: 21237638]
Tanaka M, Fernandez-del Castillo C, Adsay V, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012; 12: 183-197. [PMID: 22687371]
Sawhney MS, Al-Bashir S, Cury MS, et al. International consensus guidelines for surgical resection of mucinous neoplasms cannot be applied to all cystic lesions of the pancreas. Clin Gastroenterol Hepatol. Dec 2009; 7: 1373-1376. [PMID: 19577006]
Shen J, Brugge WR, Dimaio CJ, Pitman MB. Molecular analysis of pancreatic cyst fluid: a comparative analysis with current practice of diagnosis. Cancer. Jun 25 2009; 117: 217-227. [PMID: 19415731]
Lee LS, Szafranska-Schwarzbach AE, Wylie D, et al. Investigating MicroRNA Expression Profiles in Pancreatic Cystic Neoplasms. Clin Transl Gastroenterol. 2014; 5: e47. [PMID: 24476997]
Park WG, Wu M, Bowen R, et al. Metabolomic-derived novel cyst fluid biomarkers for pancreatic cysts: glucose and kynurenine. Gastrointest Endosc. 2013; 78: 295-302 e292. [PMID: 23566642]
Copyright (c) 2014 Linda S Lee, Leona A Doyle, Jeffrey Houghton, Sachin Sah, Andrew M Bellizzi, Anna E Szafranska-Schwarzbach, James R Conner, Vivek Kadiyala, Shadeah L Suleiman, Peter A Banks, Bernard F Andruss, Darwin L Conwell

This work is licensed under a Creative Commons Attribution 4.0 International License.
As a member of Publisher International Linking Association, PILA, iMedPub Group’s JOP follows the Creative Commons Attribution License and Scholars Open Access publishing policies. Journal of the Pancreas is the Council Contributor Member of Council of Science Editors (CSE) and following the CSE slogan Education, Ethics, and Evidence for Editors.