A Lower Cyst Fluid CEA Cut-Off Increases Diagnostic Accuracy in Identifying Mucinous Pancreatic Cystic Lesions
Abstract
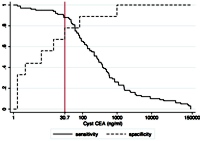
Context Carcinoembryonic antigen analysis of pancreatic cyst fluid is the tumor marker of choice for preoperatively differentiating mucinous from non-mucinous cystic lesions. Objective We aim to determine the most accurate cyst carcinoembryonic antigen cut-off value for distinguishing mucinous cysts from non-mucinous cysts with a focus on discriminating intraductal papillary mucinous neoplasms. Methods The results of pancreatic cyst aspiration carcinoembryonic antigen levels from a single center were retrospectively collected and evaluated for a diagnosis of a mucinous cyst and an assessment of malignancy using surgical histology as the diagnostic standard in 86 patients. Results The median cyst carcinoembryonic antigen level (ng/mL) was significantly higher in mucinous cysts compared with non-mucinous cysts (218 vs. 4.4; P=0.0006) and in intraductal papillary mucinous neoplasms compared with non-mucinous cysts (135 vs. 4.4; P=0.0027). A cyst carcinoembryonic antigen cut-off of 30.7 ng/mL was most accurate (87.2%) for differentiating mucinous from non-mucinous cysts and specifically for differentiating intraductal papillary mucinous neoplasms from non-mucinous cysts (82.7%). Cyst carcinoembryonic antigen levels were not significantly different between malignant and non-malignant mucinous cysts (68.5 vs. 238.1; P=0.51). Conclusions Pancreatic cyst fluid carcinoembryonic antigen can accurately differentiate histologically verified mucinous lesions, including intraductal papillary mucinous neoplasms, from non-mucinous lesions with an optimal cut-off that is much lower than previously reported values. Cyst carcinoembryonic antigen levels are not a reliable predictor of malignancy.
Image: Sensitivity and specificity curves of cyst fluid CEA levels for differentiating mucinous from non-mucinous cysts.
Downloads
References
Fernández-del Castillo C, Targarona J, Thayer SP, Rattner DW, Brugge WR, Warshaw AL. Incidental pancreatic cysts: clinicopathologic characteristics and comparison with symptomatic patients. Arch Surg 2003; 138:427–434. [PMID: 12686529].
Laffan TA, Horton KM, Klein AP, Berlanstein B, Siegelman SS, Kawamoto S, et al. Prevalence of unsuspected pancreatic cysts on MDCT. Am J Roentgenol 2008; 191:802–807. [PMID: 18716113].
Spinelli KS, Fromwiller TE, Daniel RA, Kiely JM, Nakeeb A, Komorowski RA, et al. Cystic pancreatic neoplasms: observe or operate. Ann Surg 2004; 239:651–659. [PMID: 15082969].
Brugge WR, Lauwers GY, Sahani D, Fernández-del Castillo C, Warshaw AL. Cystic neoplasms of the pancreas. N Engl J Med 2004; 351:1218–1226. [PMID: 15371579].
Pyke CM, van Heerden JA, Colby TV, Sarr MG, Weaver AL. The spectrum of serous cystadenoma of the pancreas: clinical, pathologic, and surgical aspects. Ann Surg 1992; 215:132–139. [PMID: 1546898].
Tanaka M, Chari S, Adsay V, Fernández-del Castillo C, Falconi M, Shimizu M, et al. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology 2006; 6:17–32. [PMID: 16327281].
Zamboni G, Scarpa A, Bogina G, Iacono C, Bassi C, Talamini G, et. al. Mucinous cystic tumors of the pancreas: clinicopathological features, prognosis, and relationship to other mucinous cystic tumors. Am J Surg Pathol 1999; 23:410–422. [PMID: 10199470].
Compagno J, Oertel JE. Mucinous cystic neoplasms of the pancreas with overt and latent malignancy (cystadenocarcinoma and cystadenoma): a clinicopathologic study of 41 cases. Am J Clin Pathol 1978; 69:573–580. [PMID: 665578].
Reddy RP, Smyrk TC, Zapiach M, Levy MJ, Pearson RK, Clain JE, et al. Pancreatic mucinous cystic neoplasm defined by ovarian stroma: demographics, clinical features, and prevalence of cancer. Clin Gastroenterol Hepatol 2004; 2:1026–1031. [PMID: 15551256].
Kim SC, Park KT, Lee YJ, Lee SS, Seo DW, Lee SK, et al. Intraductal papillary mucinous neoplasm of the pancreas: clinical characteristics and treatment outcomes of 118 consecutive patients from a single center. J Hepatobiliary Pancreat Surg 2008; 15:183–188. [PMID: 18392712].
Nagai K, Doi R, Kida A, Kami K, Kawaguchi Y, Ito T, et al. Intraductal papillary mucinous neoplasms of the pancreas: clinicopathologic characteristics and long-term follow-up after resection. World J. Surg. 2008; 32(2):271–278. [PMID: 18027021].
Mimura T, Masuda A, Matsumoto I, Shiomi H, Yoshida S, Sugimoto M, et al. Predictors of malignant intraductal papillary mucinous neoplasm of the pancreas. J Clin Gastroenterol 2010; 44:e224–229. [PMID: 20453661].
Bournet B, Kirzin S, Carrere N, Portier G, Otal P, Selves J, et al. Clinical fate of branch duct and mixed forms of intraductal papillary mucinous neoplasia of the pancreas. J Gastroenterol Hepatol 2009; 24:1211–1217. [PMID: 19476563].
Nara S, Onaya H, Hiraoka N, Shimada K, Sano T, Sakamoto Y, et al. Preoperative evaluation of invasive and noninvasive intraductal papillary-mucinous neoplasms of the pancreas: clinical, radiological, and pathological analysis of 123 cases. Pancreas 2009; 38:8–16. [PMID: 18665010].
Crippa S, Salvia R, Warshaw AL, Dominguez I, Bassi C, Falconi M, et al. Mucinous cystic neoplasm of the pancreas is not an aggressive entity. Ann Surg 2008; 247:571–579. [PMID: 18362619].
Tanaka M, Fernández-del Castillo C, Adsay V, Chari S, Falconi M, Jang JY, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology 2012; 12:183–197. [PMID: 22687371].
Fisher WE, Hodges SE, Yagnik V, Morón FE, Wu MF, Hilsenbeck SG, et al. Accuracy of CT in predicting malignant potential of cystic pancreatic neoplasms. HPB 2008; 10:483–490. [PMID: 19088937].
Le Borgne J, de Calan L, Partensky C. Cystadenomas and cystadenocarcinomas of the pancreas: A multiinstitutional retrospective study of 398 cases. Ann Surg 1999; 230:152-161. [PMID: 10450728].
Bassi C, Salvia R, Molinari E, Biasutti C, Falconi M, Pederzoli P. Management of 100 consecutive cases of pancreatic serous cystadenoma: wait for symptoms and see at imaging or vice versa? World J Surg 2003; 27:319–323. [PMID: 12607059].
Procacci C, Biasiutti C, Carbognin G, Accordini S, Bicego E, Guarise A, et al. Characterization of cystic tumors of the pancreas: CT accuracy. J Comput Assist Tomogr 1999; 23:906–912. [PMID: 10589565].
Kerlin DL, Frey CF, Bodai BI, Twomey PL, Ruebner B. Cystic neoplasms of the pancreas. Surg Gynecol Obstet 1987; 165:475–478. [PMID: 2446398].
Lewandrowski KB, Southern JF, Pins MR, Compton CC, Warshaw AL. Cyst fluid analysis in the differential diagnosis of pancreatic cysts: a comparison of pseudocysts, serous cystadenomas, mucinous cystic neoplasms, and mucinous cystadenocarcinoma. Ann Surg 1993; 217:41–47. [PMID: 8424699].
Frossard JL, Amouyal P, Amouyal G, Palazzo L, Amaris J, Soldan M, et al. Performance of endosonography-guided fine needle aspiration and biopsy in the diagnosis of pancreatic cystic lesions. Am J Gastroenterol 2003; 98:1516–1524. [PMID: 12873573].
Sand JA, Hyoty MK, Mattila J, Dagorn JC, Nordback IH. Clinical assessment compared with cyst fluid analysis in the differential diagnosis of cystic lesions in the pancreas. Surgery 1996; 119:275–280. [PMID: 8619182].
Sperti C, Pasquali C, Guolo P, Polverosi R, Liessi G, Pedrazzoli S. Serum tumor markers and cyst fluid analysis are useful for the diagnosis of pancreatic cystic tumors. Cancer 1996; 78:237–243. [PMID: 8673998].
Al-Haddad M, DeWitt J, Sherman S, Schmidt CM, LeBlanc JK, McHenry L, et al. Performance characteristics of molecular (DNA) analysis for the diagnosis of mucinous pancreatic cysts. Gastrointest Endosc 2014; 79:79–87. [PMID: 23845445].
Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. WHO classification of tumours of the digestive system. 4th ed Lyon: IARC Press, 2010.
Sawhney MS, Devarajan S, O'Farrel P, Cury MS, Kundu R, Vollmer CM, et al. Comparison of carcinoembryonic antigen and molecular analysis in pancreatic cyst fluid. Gastrointest Endosc 2009; 69: 1106–1110. [PMID: 19249035].
Khalid A, Zahid M, Finkelstein SD, LeBlanc JK, Kaushik N, Ahmad N, et al. Pancreatic cyst fluid DNA analysis in evaluating pancreatic cysts: a report of the PANDA study. Gastrointest Endosc 2009; 69:1095–1102. [PMID: 19152896].
van der Waaij LA, van Dullemen HM, Porte RJ. Cyst fluid analysis in the differential diagnosis of pancreatic cystic lesions: a pooled analysis. Gastrointest Endosc 2005; 62:383–389. [PMID: 16111956].
Brugge WR, Lewandrowski K, Lee-Lewandrowski E, Centeno BA, Szydlo T, Regan S, et al. Diagnosis of pancreatic cystic neoplasms: a report of the cooperative pancreatic cyst study. Gastroenterology 2004; 126:1330–1336. [PMID: 15131794].
Cizginer S, Turner BG, Bilge AR, Karaca C, Pitman MB, Brugge WR. Cyst fluid carcinoembryonic antigen is an accurate diagnostic marker of pancreatic mucinous cysts. Pancreas 2011; 40:1024–1028. [PMID: 21775920].
Shami VM, Sundaram V, Stelow EB, Conaway M, Moskaluk CA, White GE, et al. The level of carcinoembryonic antigen and the presence of mucin as predictors of cystic pancreatic mucinous neoplasia. Pancreas 2007; 34:466–469. [PMID: 17446847].
Schmidt CM, White PB, Waters JA, Yiannoutsos CT, Cummings OW, Baker M, et al. Intraductal papillary mucinous neoplasms: predictors of malignant and invasive pathology. Ann Surg 2007; 246:644-654. [PMID: 17893501].
Ohno E, Hirooka Y, Itoh A, Ishigami M, Katano Y, Ohmiya N, et al. Intraductal papillary mucinous neoplasms of the pancreas: differentiation of malignant and benign tumors by endoscopic ultrasound findings of mural nodules. Ann Surg 2009; 249:628–634. [PMID: 19300203].
Lee SY, Lee KT, Lee JK, Jeon YH, Choi D, Lim JH, et al. Long-term follow up results of intraductal papillary mucinous tumors of pancreas. J Gastroenterol Hepatol 2005; 20:1379–1384. [PMID: 16105124].
Singhi AD, Chu LC, Tatsas AD, Shi C, Ellison TA, Fishman EK, et al. Cystic pancreatic neuroendocrine tumors: a clinicopathologic study. Am J Surg Pathol 2012; 36:1666–1673. [PMID: 23073325].
Curry CA, Eng J, Horton KM, Urban B, Siegelman S, Kuszyk BS, Fishman EK. CT of primary cystic pancreatic neoplasms: can CT be used for patient triage and treatment? Am J Roentgenol 2000; 175:99–103. [PMID: 10882255].
Minami M, Itai Y, Ohtomo K, Yoshida H, Yoshikawa K, Iio M. Cystic neoplasms of the pancreas: comparison of MR imaging with CT. Radiology 1989; 171:53–56. [PMID: 2928546].
Koito K, Namieno T, Nagakawa T, Shyonai T, Hirokawa N, Morita K. Solitary cystic tumor of the pancreas: EUS-pathologic correlation. Gastrointest Endosc 1997; 45:268–276. [PMID: 9087833].
Ahmad NA, Kochman ML, Brensinger C, Brugge WR, Faigel DO, Gress FG, et al. Interobserver agreement among endosonographers for the diagnosis of neoplastic versus non-neoplastic pancreatic cystic lesions. Gastrointest Endosc 2003; 58:59–64. [PMID: 12838222].
O’Toole D, Palazzo L, Hammel P, Ben Yaghlene L, Couvelard A, Felce-Dachez M, et al. Macrocystic pancreatic cystadenoma: the role of EUS and cyst fluid analysis in distinguishing mucinous and serous lesions. Gastrointest Endosc 2004; 59:823–829. [PMID: 15173795].
Helpap B, Vogel J. Immunohistochemical studies on cystic pancreatic neoplasms. Pathol Res Pract 1988; 184:39–45. [PMID: 2852801].
Shen J, Brugge WR, DiMaio CJ, Pitman MB. Molecular analysis of pancreatic cyst fluid. Cancer Cytopathol 2009; 117:217–227. [PMID: 19415731].
Wu J, Matthaei H, Maitra A, Dal Molin M, Wood LD, Eshleman JR, et al. Recurrent GNAS mutations define an unexpected pathway for pancreatic cyst development. Sci Transl Med 2011; 3:92ra66. [PMID: 21775669].
Layfield LJ, Eyha H, Filie AC, Hruban RH, Jhala N, Joseph L, et al. Utilization of ancillary studies in the cytologic diagnosis of biliary and pancreatic lesions: the Papanicolaou Society of Cytopathology guidelines for pancreatobiliary cytology. Diagn Cytopathol 2014; 42:351-362. [PMID: 24639398].
Sreenarasimhaiah J, Lara LF, Jazrawi SF, Barnett CC, Tang SJ. A comparative analysis of pancreas cyst fluid CEA and histology with DNA mutational analysis in the detection of mucin producing or malignant cysts. JOP 2009; 10:163–168. [PMID: 19287110].
Napoleon B, Pujol B, Lemaistre AI, Caillol F, Lucidarme D, Filoche B, et al. In Vivo Characterization of Pancreatic Serous Cystadenomas by Needle-Based Confocal Laser Endomicroscopy (nCLE). Intra and Inter Observer Agreement - Contact Study. Gastroenterology 2013; 144:S–797.
Konda VJ, Meining A, Jamil AH, Giovannini M, Hwang JH, Wallace MB, et al. A pilot study of in vivo identification of pancreatic cystic neoplasms with needle-based confocal laser endomicroscopy under endosonographic guidance. Endoscopy 2013; 45:1006–1013. [PMID: 24163192].
Hammel P, Levy P, Voitot H, Levy M, Vilgrain V, Zins M, et al. Preoperative cyst fluid analysis is useful for the differential diagnosis of cystic lesions of the pancreas. Gastroenterology 1995; 108:1230–1235. [PMID: 7535275].
Repák R, Rejchrt S, Bártová J, Malírová E, Tycová V, Bures J. Endoscopic ultrasonography (EUS) and EUS-guided fine-needle aspiration with cyst fluid analysis in pancreatic cystic neoplasms. Hepatogastroenterology 2009; 56:629–635. [PMID: 19621669].
Snozek CL, Mascarenhas RC, O’Kane DJ. Use of cyst fluid CEA, CA19-9, and amylase for evaluation of pancreatic lesions. Clin Biochem 2009; 42:1585–1588. [PMID: 19576876].
Maire F, Voitot H, Aubert A, Palazzo L, O’Toole D, Couvelard A, et al. Intraductal papillary mucinous neoplasms of the pancreas: performance of pancreatic fluid analysis for positive diagnosis and the prediction of malignancy. Am. J. Gastroenterol 2008; 103:2871–2877. [PMID: 18775021].
Linder JD, Geenen JE, Catalano MF. Cyst fluid analysis obtained by EUS-guided FNA in the evaluation of discrete cystic neoplasms of the pancreas: a prospective single-center experience. Gastrointest Endosc 2006; 64:697–702. [PMID: 17055859].
Buscaglia JM, Giday SA, Kantsevoy SV, Jaqannath SB, Magno P, Wolfgang CL, et al. Patient- and cyst-related factors for improved prediction of malignancy within cystic lesions of the pancreas. Pancreatology 2009; 9:631–638. [PMID: 19657218].
Nagula S, Kennedy T, Schattner MA, Brennan MF, Gerdes H, Markowitz AJ, et al. Evaluation of cyst fluid CEA analysis in the diagnosis of mucinous cysts of the pancreas. J. Gastrointest Surg 2010; 14: 1997–2003. [PMID: 20658204].
Copyright (c) 2015 David X Jin, Aaron J Small, Charles M Vollmer, Nirag Jhala, Emma E Furth, Gregory G Ginsberg, Michael L Kochman, Nuzhat A Ahmad, Vinay Chandrasekhara

This work is licensed under a Creative Commons Attribution 4.0 International License.
As a member of Publisher International Linking Association, PILA, iMedPub Group’s JOP follows the Creative Commons Attribution License and Scholars Open Access publishing policies. Journal of the Pancreas is the Council Contributor Member of Council of Science Editors (CSE) and following the CSE slogan Education, Ethics, and Evidence for Editors.