An Immunocytochemical Profile of the Endocrine Pancreas Using an Occlusive Duct Ligation Model
Abstract
Context Ligation of the pancreatic duct, distally to its confluence into the bile duct has been shown to induce endocrine tissue regeneration. The surplus endocrine tissue formed is presumed to be able to replace pathologically and/or experimentally compromised tissue. Objective This is a quantitative study on the histology of duct ligated pancreas employing immunocytochemistry and computerised morphometry. Interventions Pancreatic duct ligation was performed on 25 groups of six normal Sprague-Dawley rats. Experimental animals were sacrificed at 12-hour intervals from day one to ten post-duct ligation and every 24 hours thereafter to day 14, the pancreas removed, fixed and processed. Six consecutive 3-6 micron serial sections were cut on a rotary hand microtome, floated onto 3-aminopropyl-trimethoxysilan coated slides and alternatively immunocytochemically stained for insulin, glucagon, pancreatic polypeptide and somatostatin. Results Pancreas transformation between days ½ and 3½ was characterised by acinar deletion and the appearance of immunoreactive cells for the primary endocrine hormones. Transdifferentiation of existing endocrine tissue saw islet insulin core cells replaced by pancreatic polypeptide- and somatostatin positive cells, glucagon deletion and random appearance of all endocrine cell types within the inter-islet interstitium by day 3½. Days 4 to 14 were characterised by cellular migration and islet reconstruction. Conclusions To date our laboratory has investigated transplantation of foetal tissue beneath the renal capsule in syngeneic, isogeneic and allogeneic normal and diabetic rats. As pancreatic duct ligation induces the development of surplus endocrine tissue our next step would be to investigate the use of ligated pancreas as a replacement for foetal tissue.
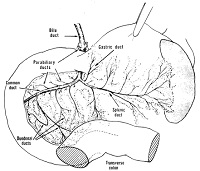
Image: Arrangement of bile and pancreatic ducts in the laboratory rat.
Downloads
References
Rosenberg L, Duguid WP. Trophic stimulation of the ductal/islet cell axis: A new approach to the treatment of diabetes. Surgery 1990; 108:191-7.
Rawdon B. Gastrointestinal hormones in birds: Morphological, chemical, and developmental aspects. J Exp Zoo 1984; 232:659-70.
Teitelman G, Lee J, Reis DJ. Differentiation of prospective mouse pancreatic islet cells during development in vitro and during regeneration. Dev Biol 1987; 120:425-33.
Walker NI. Ultra structure of the rat pancreas after experimental duct ligation. I. The role of apoptosis and intraepithelial macrophages in acinar cell deletion. Am J Pathol 1987; 126:439-51.
Andrew A, Rawdon BB, Kramer B. Differentiation of ectopic endocrine cells from avian gastric and pancreatic endoderm. Cell Diff 1988; 22:135-44.
Herrera, PL, Huarte J, Sanvito F, Meda, P, Orci L, Vassali JD. Embryogenesis of the murine endocrine pancreas; early expression of pancreatic polypeptide gene. Development 1991; 113:1257-65.
Wolfe-Coote SA, Chapman C, Louw J. Interesting endocrine cell distributions in the developing non-human primate pancreas. Histochem J 1992; 24:484-5.
Bonner-Weir S, Baxter LA, Schuppin GT, Smith F. A second pathway for regeneration of adult exocrine and endocrine pancreas: A possible recapitulation of embryonic development. Diabetes 1993; 42:1715-20.
Kaung HL. Growth dynamics of pancreatic islet cell populations during foetal and neonatal development of the rat. Dev Dyn 1994; 200:163-75.
Bouwens L, Lu WG, De Krijger R. Proliferation and differentiation in the human foetal endocrine pancreas. Diabetologia 1997; 40:398-404.
McEvoy RC. Changes in the volumes of the A, B and D-cell populations in the pancreatic islets during the postnatal development of the rat. Diabetes 1981; 30:813-7.
Githens S. Differentiation and development of the pancreas in animals. In: Lang V, Go W, eds. The Pancreas: Biology, Pathobiology and Disease, 2nd Ed.. New York: Raven Press, Ltd. 1993: 21-55.
Brockenbrough JS, Weir GC, Bonner-Weir S. Discordance of exocrine and endocrine growth after 90% pancreatectomy in rats. Diabetes 1988; 37:232-6.
Gu D, Arnush M, Sarvetnick N. Endocrine/exocrine intermediate cells in streptozotocin-treated Ins-IFN-gamma transgenic mice. Pancreas 1997; 15:246-50.
Wang R, Kloppel G, Bouwens L. Duct to islet cell differentiation and islet growth in the pancreas of duct ligated adult rats. Diabetologia 1995; 38:1405-11.
Abe K, Watanabe S. Apoptosis of mouse pancreatic acinar cells after duct ligation. Arch Histol Cytol 1995; 58:221-9.
Gukovskaya AS, Perkins P, Zaninovic V, Sandoval D, Rutherford R, Fitzsimmons T, et al. Mechanisms of cell death after pancreatic duct obstruction in the opossum on the rat. Gastroenterology 1996; 110:875-84.
Bertelli E, Bendayan M. Intermediate endocrine-avinar pancreatic cells in duct ligation conditions. Am J Physiol 1997; 273:C1641-9.
Rosenberg L. Induction of islet cell neogenesis in the adult pancreas: the partial duct obstruction model. Microsc Res Tech 1998; 43:337-46.
Ferrand N, Astesano A, Phan HH, Lelong C, Rosselin G. Dynamics of pancreatic cell growth and differentiation during diabetes reversion in STZ-treated newborn rats. Am J Physiol 1995; 269:C1250-64.
Zenilman ME, Perfetti R, Swinson K, Magnuson T, Schuldiner AR. Pancreatic regeneration (reg) gene expression in a rat model of islet hyperplasia. Surgery 1996; 199:576-84.
Gittes GK. Studies of early events in pancreatic organogenesis. Ann NY Acad Sci 1994; 733:68-74.
Bouwens L, Wang R, De Blay E, Pipeleers DG. Kloppel G. Cytokeratins as markers of ductal cell differentiation and islet neogenesis in the neonatal rat pancreas. Diabetes 1994; 43:1279-83.
Jackerott M, Larsson LI. Immunocytochemical localisation of the NPY/PYY Y1 receptor in the developing pancreas. Endocrinology 1997; 138:5013-8.
Larsson LI. On the development of the islets of Langerhans. Microsc Res Tech 1998; 43:284-291.
Upchurch BH, Aponte GW, Leiter AB. Expression of peptide YY in all four islet cell types in the developing mouse pancreas suggests a common peptide YY-producing progenitor. Development 1994; 120:245-52.
Scarpelli DG, Rao MS. Differentiation of regenerating pancreatic cells into hepatocyte-like cells. Proc Natl Acad Sci USA 1980; 78:2577-81.
Yamaguchi Y, Matsuno K, Goto M, Ogawa M. In situ kinetics of acinar, duct and inflammatory cells in duct ligation-induced pancreatitis in rats. Gastroenterology 1993; 105:1498-506.
Idezuki Y, Goetz FC, Lillehei RC. Late effect of pancreatic duct ligation on beta-cell function. Am J Surg 1997; 117:33-9.
Edstrom C, Falkmer S. Pancreatic morphology and blood glucose level in rats at various intervals after duct ligation. Virchows Arch A Pathol Pathol Anat 1968; 345:139-53.
Teitelman G. On the origin of pancreatic endocrine cells, proliferation and neoplastic transformation. Tumor Biology 1993; 14:167-73.
Guesdon JL, Terugnck T, Avrameas S. The use of avidin-biotin interaction in immuno-enzymatic techniques. J Histochem Cytochem 1979; 27:1131-9.
Louw J. The Establishment of Baseline Parameters of the Vervet Monkey Endocrine Pancreas as a Model to Investigate possible therapies for Diabetes. PhD Thesis, University of Western Cape ,Western Cape, South Africa, 1995.
Wolfe-Coote SA, Louw J, Woodroof CW, Heydenrych JJ, Du Toit DF. Induction of cell proliferation an differentiation in the pancreas of the adult Vervet Monkey (Cercopithecus Aethiops). Pancreas 1998; 16:129-33.
McEvoy RC, Hegre OD. Morphometric quantitation of the pancreatic insulin-, glucagon-, and somatostatin-positive cell populations in normal and alloxan-diabetic rats. Diabetes 1997; 26:1140-6.
Melmed RN, Benitez CJ, Hol, SJ. Intermediate cells of the pancreas. J Cell Science 1972; 11:449-75.
Bouwens L, Braet F, Heimberg H. Identification of rat pancreatic duct cells by their expression of cytokeratins 7, 19 and 20 in vivo and after isolation and culture. J Histochem Cytochem 1995; 43:245-53.
Bouwens L, De Blay E. Islet morphogenesis and stem cell markers in rat pancreas. J Histochem Cytochem 1996; 44:947-51.
Du Toit DF, Muller CJF, Page BJ, Louw J. Foetal rat pancreatic transplantation: post-transplantation development of foetal pancreatic iso- and allografts and suppression of rejection with mycophenolate mofetil (MMF) and cyclosporine based immunesuppression. Micr Res Tech 1998; 43:347-55.
Copyright (c) 2000 Benedict J Page, Don F du Toit, Christo JF Muller, Johannes Mattysen, Romeo Lyners

This work is licensed under a Creative Commons Attribution 4.0 International License.
As a member of Publisher International Linking Association, PILA, iMedPub Group’s JOP follows the Creative Commons Attribution License and Scholars Open Access publishing policies. Journal of the Pancreas is the Council Contributor Member of Council of Science Editors (CSE) and following the CSE slogan Education, Ethics, and Evidence for Editors.