Laboratory
Abstract
No abstract available.
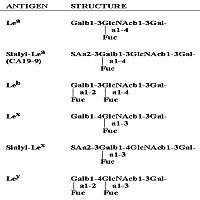
Image: Blood group related antigens.
Downloads
References
Plebani M, Basso D, Panozzo MP, Fogar P, Del Favero G, Naccarato R. Tumor markers in the diagnosis, monitoring and therapy of pancreatic cancer: state of the art. Int J Biol Marker 1995; 10:189-99.
Lamerz R. Role of tumor markers, cytogenetics. Ann Oncol 1999; 10(Suppl. 4):S145-9.
Brockhausen I. Pathways of O-glycan biosynthesis in cancer cells. Biochem Biophys Acta 1999; 1473:67-95.
Basso D, Fabris C, Meggiato T, Del Favero G, Fogar P, Panozzo MP, et al. Serum levels of CA 19-9 and tissue polypeptide antigen (TPA) are related to the presence of a benign extra-hepatic cholestasis. Med Sci Res 1989; 17:13-4.
Halm U, Schumann T, Schiefke I, Witzigmann H, Mossner J, Keim V. Decrease of CA 19-9 during chemotherapy with gemcitabine predicts survival time in patients with advanced pancreatic cancer. Br J Cancer 2000; 82:1013-6.
Friess H, Kleef J, Kork M, Buchler MW. Molecular aspects of pancreatic cancer and future perspectives. Dig Surg 1999; 16:281-90.
Lemoine NR, Jain S, Hughes CM, Staddon SL, Maillet B, Hall PA, et al. Ki-ras oncogene activation in preinvasive pancreatic cancer. Gastroenterology 1992; 102:230-6.
Hruban RH, van Mansfeld AD, Offerhaus GJ, van Weering DH, Allison DC, Goodman SN, et al. K-ras oncogene activation in adenocarcinoma of the human pancreas. A study of 82 carcinomas using a combination of mutant-enriched polymerase chain reaction analysis and allele-specific oligonucleotide hybridization. Am J Pathol 1993; 143:545-54.
Schaeffer BK, Glasner S, Kuhlmann E, Myles JL, Longnecker DS. Mutated c-Ki-ras in small pancreatic adenocarcinoma. Pancreas 1994; 9:161-5.
Kern SE. Advances from genetic clues in pancreatic cancer. Current Opinion in Oncology 1998; 10: 4-80.
Yamaguchi Y, Watanabe H, Yrdiran S, Ohtsubo K, Motoo Y, Okai T, Sawabu N. Detection of mutations of p53 tumor suppressor gene in pancreatic juice and its application to the diagnosis of patients with pancreatic cancer: comparison with K-ras mutation. Clin Cancer Res 1999; 5:1147-53.
Dai JL, Schutte M, Bansal RK, Wilentz RE, Sugar AY, Kern SE. Transforming growth factor-beta responsiveness in DPC4/SMAD4-null cancer cells. Mol Carcinogenesis 1999; 26:37-43.
Schutte M. DPC4/SMAD4 gene alterations in human cancer, and their functional implications. Ann Oncol 1999; 10(Suppl. 4):S56-9.
Kondo H, Sugano K, Fukayama N, Kyogoku A, Nose H, Shimada K, et al. Detection of point mutations in the K-ras oncogene at codon 12 in pure pancreatic juice for diagnosis of pancreatic carcinoma. Cancer 1994; 73:1589-94.
Caldas C, Hahn SA, Hruban RH, Redston MS, Yeo CJ, Kern SE. Detection of K-ras mutations in the stool(s) of patients with pancreatic adenocarcinoma and pancreatic ductal hyperplasia. Cancer Res 1994;54:3568-73.
Abbruzzese JL, Evans DB, Raijman I, Larry L, King T, Leach SD, et al. Detection of mutated c-Ki-ras in the bile of patients with pancreatic cancer. Anticancer Res 1997; 17:795-801.
Wilentz RE, Chung CH, Sturm PD, Musler A, Sohn TA, Offerhaus GJ, et al. K-ras mutations in the duodenal fluid of patients with pancreatic carcinoma. Cancer 1998; 82:96-103.
Ito R, Tamura K, Ashida H, Nishiwaki M, Nishioka A, Yamamoto Y, et al. Usefulness of K-ras gene mutation at codon 12 in bile for diagnosing biliary strictures. Int J Oncol 1998;12:1019-23.
Mulcahy HE, Lyautey J, Lederrey C, Qi Chen X, Anker P, Alstead EM, et al. A prospective study of K-ras mutations in the plasma of pancreatic cancer patients. Clin Cancer Res 1998 ;4:271-5.
Shibata K, Mori M, Kitano S, Akiyoshi T. Detection of ras gene mutations in peripheral blood of carcinoma patients using CD45 immunomagnetic separation and nested mutant allele specific amplification. Int J Oncol 1998;12:1333-8.
Mulcahy H, Farthing MJG. Diagnosis of pancreatico-biliary malignancy: detection of gene mutations in plasma and stool. Annals Oncol 1999; 10:S114-7.
Gerhard M, Juhl H, Kalthoff H, Schreiber HW, Wagener C, Neumaier M. Specific detection of carcinoembryonic antigen-expressing tumor cells in bone marrow aspirates by polymerase chain reaction. J Clin Oncol 1994; 12:725-9.
Funaki NO, Tanaka J, Kasamatsu T, Ohshio G, Hosotani R, Okino T, Imamura M. Identification of carcinoembryonic antigen mRNA in circulating peripheral blood of pancreatic carcinoma and gastric carcinoma patients. Life Sci 1996; 59:2187-99.
Funaki NO, Tanaka J, Hosotani R, Kogire M, Suwa H, Imamura M. Quantitative analysis of carcinoembryonic antigen messenger RNA in peripheral venous blood and portal blood of patients with pancreatic ductal adenocarcinoma. Clin Cancer Res 1998; 4:855-60.
Thorban S, Roder JD, Siewert JR: Detection of micrometastasis in bone marrow of pancreatic cancer patients. Ann Oncol 1999; 10(Suppl. 4):S111-3.
Miyazono F, Takao S, Natsugoe S, Uchikura K, Kijima F, Aridome K, et al. Molecular detection of circulating cancer cells during surgery in patients with biliary-pancreatic cancer. Am J Surg 1999; 177:475-9.
Roder JD, Thorban S, Pantel K, Siewert JR. Micrometastases in bone marrow: prognostic indicators for pancreatic cancer. World J Surg 1999; 23:888-91.
Liefers GJ, Cleton-Jansen AM, van de Velde CJ, Hermans J, van Krieken JH, Cornelisse CJ, Tollenaar RA. Micrometastases and survival in stage II colorectal cancer. N Engl J Med 1998; 339:223-8.
Rosa JA, Van Linda BM, Abourizk NN. New-onset diabetes mellitus as a harbinger of pancreatic carcinoma. J Clin Gastroenterol 1989; 11:211-5.
Permert J, Larsson J, Ihse I, Pour PM. Diagnosis of pancreatic cancer. Alteration of glucose metabolism. Int J Pancreatol 1991; 9:113-7.
Fogar P, Basso D, Panozzo MP, Del Favero G, Briani G, Fabris C, et al.: C-peptide pattern in patients with pancreatic cancer. Anticancer Res 1993; 13:2577-80.
Permert J, Ihse I, Jorfeldt L, von Schenck H, Arnqvist H J, Larsson J. Pancreatic cancer is associated with impaired glucose metabolism. Eur J Surg 1993; 159:101-7.
Fogar P, Pasquali C, Basso D, Sperti C, Panozzo MP, Tessari G, et al.: Diabetes mellitus in pancreatic cancer follow-up. Anticancer Res 1994; 14:2827-30.
Basso D, Plebani M, Fogar P, Del Favero G, Briani G, Meggiato T, et al. Beta cell function in pancreatic adenocarcinoma. Pancreas 1994; 3:332-5.
Wang F, Larsson J, Abdiu A, Gasslander T, Westermark P, Adrian TE, Permert J. Dissociated secretion of islet amyloid polypeptide and insulin in serum-free culture media conditioned by human pancreatic adenocarcinoma cell lines. Int J Pancreatol 1997; 21:157-64.
Permert J, Adrian T E, Jacobsson P, Jorfelt Fruin B, Larsson J. Is profound insulin resistance in patients with pancreatic cancer caused by a tumor-associated factor? Am J Surg 1993; 165:61-7.
Basso D, Brigato L, Veronesi A, Panozzo MP, Amadori A, Plebani M. The pancreatic cancer cell line MIA PaCa 2 produces one or more factors able to induce hyperglycemia in SCID mice. Anticancer Res 1995; 15:132-8.
Basso D, Valerio A, Brigato L, Panozzo MP, Miola M, Lucca T, et al. An unidentified pancreatic cancer cell product alters some intracellular pathways of glucose metabolism in isolated rat hepatocytes. Pancreas 1997; 15:132-8.
Valerio A, Basso D, Brigato L, Ceolotto G, Baldo G, Tiengo A, Plebani M. Glucose metabolic alterations in isolated and perfused rat hepatocytes induced by pancreatic cancer conditioned medium: a low molecular weight factor possibly involved. Biochem Biophys Res Commun 1999; 257:622-8.
Ding X, Flatt PR, Permert J, Adrian TE. Pancreatic cancer selectively stimulates islet b cells to secrete amylin. Gastroenterology 1998; 114:130-8.
Copyright (c) 2000 Daniela Basso

This work is licensed under a Creative Commons Attribution 4.0 International License.
As a member of Publisher International Linking Association, PILA, iMedPub Group’s JOP follows the Creative Commons Attribution License and Scholars Open Access publishing policies. Journal of the Pancreas is the Council Contributor Member of Council of Science Editors (CSE) and following the CSE slogan Education, Ethics, and Evidence for Editors.