Biomarkers and Pharmacogenetics in Pancreatic Cancer
Abstract
Appropriate identification and validation of biomarkers as well as pharmacogenetics are important in formulating patient-oriented, individualized chemotherapy or biological therapy in cancer patients. These markers can be especially valuable in pancreatic cancer, where high mortality and complex disease biology are frequently encountered. Recently, several advances have been made to further our knowledge in this specific area of pancreatic cancer. In the 2011 American Society of Clinical Oncology (ASCO) Annual Meeting, researchers have presented several interesting results in biomarkers development: the identifications of 9 single nucleotide polymorphisms (SNPs) that is associated with positive efficacy of gemcitabine (Abstract #4022); the introduction of circulating tumor cells as a prognostic markers in pancreatic adenocarcinoma (Abstract #e14657); the re-affirmation of plasma cytidine deaminase (CDA) as a positive predictive markers for gemcitabine efficacy, as well as the postulations that CDA*3 as a potential genotype marker to predict gemcitabine responses (Abstract #e14645); and finally the retrospective tumor tissues analysis in the Arbeitsgemeinschaft Internistische Onkologie (AIO) trial in an attempt for epidermal growth factor receptor (EGFR) pathway biomarker identifications (Abstract #4047).
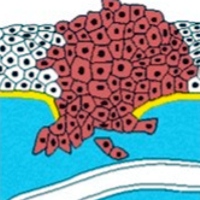
Image: Circulating tumor cells and process of metastasis (Detail. Copyright ©2002 from Molecular Biology of the Cell by Alberts et al. New York, NY, USA: Garland Sciences, 2002:132.
Downloads
References
Kim R. FOLFIRINOX: a new standard treatment for advanced pancreatic cancer? Lancet Oncol 2011; 12(1):8-9.
Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011; 364:1817-25.
McWilliams R, Bamlet W, Matsumoto M, et al. Genome-wide interaction study of gemcitabine treatment and genotype on survival in pancreatic cancer. J Clin Oncol 2011; 29(Suppl.):4022.
Fidler IJ. The pathogenesis of cancer metastasis: the 'seed and soil' hypothesis revisited. Nat Rev Cancer 2003; 3(6):453-8.
Allard WJ, Matera J, Miller MC, et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res 2004; 10(20):6897-6904.
Nagrath S, Sequist LV, Maheswaran S, et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature 2007; 450(7173):1235-1239.
Stott SL, Hsu CH, Tsukrov DI, et al. Isolation of circulating tumor cells using a microvortex-generating herringbone-chip. Proc Natl Acad Sci U S A 2010; 107(43):18392-18397.
Khan MS, Tsigani T, Rashid M, et al. Circulating tumor cells and EpCAM expression in neuroendocrine tumors. Clin Cancer Res 2011; 17(2):337-345.
de Albuquerque A, Kubisch I, Ernst D, Breier G, Kaul S, Fersis N. Prognostic significance of multimarker circulating tumor cells analysis in patients with advanced pancreatic cancer. J Clin Oncol 2011; 29(Suppl.):e14657.
Gilbert JA, Salavaggione OE, Ji Y, et al. Gemcitabine pharmacogenomics: cytidine deaminase and deoxycytidylate deaminase gene resequencing and functional genomics. Clin Cancer Res 2006; 12(6):1794-1803.
Sugiyama E, Kaniwa N, Kim SR, et al. Pharmacokinetics of gemcitabine in Japanese cancer patients: the impact of a cytidine deaminase polymorphism. J Clin Oncol 2007; 25(1):32-42.
Ueno H, Kaniwa N, Okusaka T, et al. Homozygous CDA*3 is a major cause of life-threatening toxicities in gemcitabine-treated Japanese cancer patients. Br J Cancer 2009; 100(6):870-873.
Ueno H, Kaniwa N, Sugiyama E, et al. Effect of cytidine deaminase (CDA)-related biomarkers on overall survival in patients with advanced pancreatic cancer receiving gemcitabine (GEM) monotherapy. J Clin Oncol 2011; 29(Suppl.):e14645.
Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 2007; 25(15):1960-1966.
Boeck SH, Vehling-Kaiser U, Waldschmidt D, Kettner E, Marten A, Winkelmann C, et al. Gemcitabine plus erlotinib (GE) followed by capecitabine (C) versus capecitabine plus erlotinib (CE) followed by gemcitabine (G) in advanced pancreatic cancer (APC): A randomized, cross-over phase III trial of the Arbeitsgemeinschaft Internistische Onkologie (AIO). J Clin Oncol 2010; 28(18 Suppl.):LBA4011.
Boeck S, Jung A, Laubender R, et al. Molecular markers of the EGFR pathway in erlotinib-treated patients with advanced pancretic cancer (APC): translational analyses of a randomised, cross-over AIO phase III trial. J Clin Oncol 2011; 29(Suppl.):4047.
Mandrekar SJ, Sargent DJ. Clinical trial designs for predictive biomarker validation: theoretical considerations and practical challenges. J Clin Oncol 2009; 27(24):4027-4034.
Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular Biology of the Cell, 4th ed. New York, NY, USA: Garland Sciences, 2002:132.
Copyright (c) 2011 Xunhai Xu, Alexios S Strimpakos, Muhammad Wasif Saif

This work is licensed under a Creative Commons Attribution 4.0 International License.
As a member of Publisher International Linking Association, PILA, iMedPub Group’s JOP follows the Creative Commons Attribution License and Scholars Open Access publishing policies. Journal of the Pancreas is the Council Contributor Member of Council of Science Editors (CSE) and following the CSE slogan Education, Ethics, and Evidence for Editors.