Updates on First-Line Treatment of Metastatic Pancreatic Adenocarcinoma
Abstract
Despite the extensive research, mounting knowledge in the cancer field and enormous investments, pancreatic cancer remains a rather incurable disease with aggressive natural course and high mortality rate. The very slow progress is a result of the complex pathogenesis of this disease, which prevents us from targeting the culprit and making a step forward. Therefore, the field is still unexplored and this is a real challenge and opportunity for new ideas and novel approaches. In this paper, we will present the most interesting studies in the first line pancreatic cancer setting, presented at the American Society of Clinical Oncology (ASCO) 2011 Annual Meeting. While there are few studies testing the role of combining the cytotoxic S-1 and gemcitabine, the majority of the studies are examining the safety and impact of adding to the classic gemcitabine treatment novel molecular agents which target key pathways or overexpressed proteins.
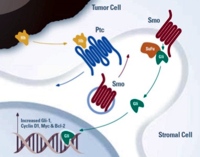
Image: The hedgehog pathway and the interaction of tumor cells with stromal cell.
Downloads
References
Strimpakos A, Saif MW, Syrigos KN. Pancreatic cancer: from molecular pathogenesis to targeted therapy. Cancer Metastasis Rev 2008; 27(3):495-522.
Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011; 364(19):1817-1825.
Strimpakos AS, Syrigos KN, Saif MW. The molecular targets for the diagnosis and treatment of pancreatic cancer. Gut Liver 2010; 4(4):433-449.
Omuro Y, Ikari T, Ishii H, Ozaka M, Suyama M, Matsumura Y, et al. A randomized phase II study of gemcitabine plus S-1 versus gemcitabine alone in patients with unresectable pancreatic cancer. J Clin Oncol 2011; 29(Suppl.):4029.
Isayama H, Nakai Y, Sasaki T, Sasahira N, Hirano K, Tsujino T, et al. The final analysis of a multicenter randomized controlled trial of gemcitabine (G) alone versus gemcitabine and S-1 combination therapy (GS) in patients with unresectable advanced pancreatic cancer (PC): GEMSAP study. J Clin Oncol 2011; 29(Suppl.):4040.
Ioka T, Ikeda M, Ohkawa S, Yanagimoto H, Fukutomi A, Sugimori K, et al. Randomized phase III study of gemcitabine plus S-1 (GS) versus S-1 versus gemcitabine (GEM) in unresectable advanced pancreatic cancer (PC) in Japan and Taiwan: GEST study. J Clin Oncol 2011; 29(Suppl.):4007.
Kim GP, Foster NR, Salim M, Flynn PJ, Moore DF, Zon R, et al. Randomized phase II trial of panitumumab, erlotinib, and gemcitabine (PGE) versus erlotinib-gemcitabine (GE) in patients with untreated, metastatic pancreatic adenocarcinoma. J Clin Oncol 2011; 29(Suppl.):4030.
Javle MM, Varadhachary GR, Fogelman DR, Shroff RT, Overman MJ, Ukegbu L, et al. Randomized phase II study of gemcitabine (G) plus anti-IGF-1R antibody MK-0646, G plus erlotinib (E) plus MK-0646 and G plus E for advanced pancreatic cancer. J Clin Oncol 2011; 29(Suppl.):4026.
Lu J, Deng H, Tang R, Hsu C, Kindler HL, Fuchs CS, et al. Exposure-response (E-R) analysis to facilitate phase III (P3) dose selection for ganitumab (GAN, AMG 479) in combination with gemcitabine (G) to treat metastatic pancreatic cancer (mPC). J Clin Oncol 2011; 29(Suppl.):4049.
McCaffery I, Tudor Y, Deng H, Tang R, Badola S, Kindler HL, et al. Effect of baseline (BL) biomarkers on overall survival (OS) in metastatic pancreatic cancer (mPC) patients (pts) treated with ganitumab (GAN; AMG 479) or placebo (P) in combination with gemcitabine (G). J Clin Oncol 2011; 29(Suppl.):4041.
Aglietta M, Barone C, Sawyer MB, Moore MJ, Miller WH, Bagal C, et al. Final toxicity results of a phase I dose-escalation trial of tremelimumab (CP-675206) in combination with gemcitabine in chemotherapy-naive patients (pts) with metastatic pancreatic cancer. J Clin Oncol 2011; 29(Suppl.):4081.
Wolpin BM, O'Reilly EM, Ko Y, Blaszkowsky LS, Rarick MU, Rocha Lima C. MS, et al. Global, multicenter, open-label, randomized phase II trial comparing gemcitabine (G) with. G plus AGS-1C4D4 (A) in patients (pts) with metastatic pancreatic cancer (mPC). J Clin Oncol 2011; 29(Suppl.):4031.
Ramanathan RK, Chadha M, Gressler V, Shah S, Loury D, Hamdy A, et al. Phase I/II pharmacokinetic and pharmacodynamic study of PCI-27483, a coagulation factor VIIa (FVIIa) inhibitor, in patients with advanced pancreatic cancer receiving treatment with gemcitabine. J Clin Oncol 2011; 29(Suppl.):e14610.
Stephenson J, Richards DA, Wolpin BM, Becerra C, Hamm JT, Messersmith WA, et al. The safety of IPI-926, a novel hedgehog pathway inhibitor, in combination with gemcitabine in patients (pts) with metastatic pancreatic cancer. J Clin Oncol 2011; 29(Suppl.):4114.
Goncalves A, Viret F, Frannois E, Dahan L, Perrier H, Lamy R, et al. BAYPAN study: A double-blind, phase III randomized trial of gemcitabine plus sorafenib versus gemcitabine plus placebo in patients with advanced pancreatic cancer. J Clin Oncol 2011; 29(Suppl.):4028.
Copyright (c) 2011 Alexios S Strimpakos, Kostas N Syrigos, Muhammad Wasif Saif

This work is licensed under a Creative Commons Attribution 4.0 International License.
As a member of Publisher International Linking Association, PILA, iMedPub Group’s JOP follows the Creative Commons Attribution License and Scholars Open Access publishing policies. Journal of the Pancreas is the Council Contributor Member of Council of Science Editors (CSE) and following the CSE slogan Education, Ethics, and Evidence for Editors.